Hydrogen 1 1 0 32 bit
Author: v | 2025-04-24

Hydrogen 1.0.0 Beta 1 (32-bit) Date released: (6 years ago) Download. Hydrogen 0.9.7 (32-bit) Date released: (7 years ago) Download. Hydrogen 0.9.7 Beta 3 (32-bit) Date released: (8 years ago) Download. Hydrogen 0.9.6 Alpha 1.
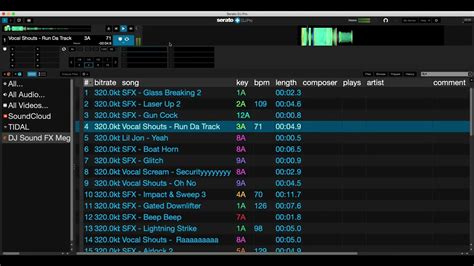
Hydrogen 1.0.0 RC 1 (32-bit) Download - FileHorse
Word EquationDihydrogen + Carbon + Dioxygen = Acetic AcidH2 + C + O2 = HCH3CO2 is a Synthesis reaction where two moles of Dihydrogen [H2], two moles of Carbon [C] and one mole of Dioxygen [O2] combine to form one mole of Acetic Acid [HCH3CO2] Reactants Dihydrogen - H2Molecular Hydrogen Hydrogen Molecule Hydrogen Hydrogen Gas Molecular Hydrogen Gas H₂ Carbon - CElement 6 Dioxygen - O2Lox Liquid Oxygen Oxygen Gas Triplet Oxygen Diatomic Oxygen Molecular Oxygen Oxygen O₂ Products Acetic Acid - HCH3CO2Ch3Cooh E260 Ethanoat Ethanoic Acid Aceticum Acidum Methanecarboxylic Acid Ethoic Acid Ethylic Acid ThermodynamicsThermodynamics of the reaction can be calculated using a lookup table.Is the Reaction Exothermic or Endothermic?H2 (g)2 mol0 kJ/mol-0 kJC (g)2 mol716.681544 kJ/mol-1433.363088 kJO2 (g)1 mol0 kJ/mol-0 kJCH3COOH (l acetic acid)1 mol-484.13064 kJ/mol-484.13064 kJΣΔH°f(reactants)1433.363088 kJΣΔH°f(products)-484.13064 kJΔH°rxn-1917.493728 kJΣΔH°f(reactants) > ΣΔH°f(products), so H2 + C + O2 = HCH3CO2 is exothermic (releases heat).Is the Reaction Exoentropic or Endoentropic?ΔS = Sproducts - Sreactants. If ΔS 0, it is endoentropic.H2 (g)2 mol130.586824 J/(mol K)-261.173648 J/KC (g)2 mol157.9865848 J/(mol K)-315.9731696 J/KO2 (g)1 mol205.028552 J/(mol K)-205.028552 J/KCH3COOH (l acetic acid)1 mol159.8288 J/(mol K)159.8288 J/KΣΔS°(reactants)782.1753696 J/KΣΔS°(products)159.8288 J/KΔS°rxn-622.3465696 J/KΣΔS°(reactants) > ΣΔS°(products), so H2 + C + O2 = HCH3CO2 is exoentropic (decrease in entropy).Is the Reaction Exergonic or Endergonic?ΔG = Gproducts - Greactants. If ΔG 0, it is endergonic.H2 (g)2 mol0 kJ/mol-0 kJC (g)2 mol671.289328 kJ/mol-1342.578656 kJO2 (g)1 mol0 kJ/mol-0 kJCH3COOH (l acetic acid)1 mol-389.9488 kJ/mol-389.9488 kJΣΔG°(reactants)1342.578656 kJΣΔG°(products)-389.9488 kJΔG°rxn-1732.527456 kJΣΔG°(reactants) > ΣΔG°(products), so H2 + C + O2 = HCH3CO2 is exergonic (releases energy). Instructions To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Compound states [like (s) (aq) or (g)]
Hydrogen 1.0.0 Beta 1 (32-bit) Download - FileHorse
MQ8-Micropython-ESP32MQ-8 Hydrogen gas sensor module for micropython. This module has been tested on ESP-32Hardware InformationThis module can be used without adding 1.47 kohm resistor to GPIO PinThe default pin used was GPIO36 which labeled as VP on ESP32 boardModify the code in MQ8.get_resistance() method to change the default PinCircuit WiringThe circuit wiring setup was tested on Espressif ESP-32 board on Pin GPIO36 (VP) using 10-bit width ADC mode. During the test Wifi connection of the boardwas still accessible. To extend the ADC GPIO connection without inferring the wifi connection please refer to ESP-32 GPIO manualHere's the connection setupSensorESP-32LabelVCC5VVinGNDGNDGNDA0GPIO36VPAdditional InformationThis software module is a part of Hydrogen Energy research in Wardana Research Group, Dept. of Mechanical Engineering, Universitas Brawijaya, Malang,IndonesiaThis module is freely available for any kind of project in research scale, Minimum Viable Product design, Prototyping, and H2 gas detectionFor Industrial Scale Hydrogen Production the use of this module and MQ-X sensor series is not recommendedResponsible PersonHead of Wardana Research Group : Prof. ING. Wardana,Ph.DSoftware Author : Dr. WIlly Satrio NResearch Manager : Dr. PurnamiContact InfoFor Research Collaboration or Project please email to wardana@ub.ac.id and alternative contact to willy13101307@gmail.comAddressesUniversitas Brawijaya : Address : Jl. Veteran, Ketawanggede, Kec. Lowokwaru, Kota Malang, Jawa Timur 65145Departement : Mechanical EngineeringTerms and ConditionsIf this module is usefull for your research project please cite one of scientific article listed below:Hydrogen production from instant noodle wastewater by organic electrocatalyst coated on PVC surface( external magnetic fields with activated carbon graphene for increasing hydrogen production in water electrolysis( role of turmeric and bicnat on hydrogen production in porous tofu waste suspension electrolysis( effect of curcumin and activated carbon catalyst enhancing hydrogen production from biomass pyrolysis( role of activated carbon in boosting the activity of clitoria ternatea powder photocatalyst for hydrogen production( effect of curcumin coated electrode on hydrogen production through water electrolysis( production by photocatalysis method of glutamic acid and activated carbon( Of Bamboo Charcoal And Fragaria Vesca Powder Photocatalysts In Hydrogen Production Via Water Splitting(DOI : 10.15587/1729-4061.2020.213277)Hydrogen 1.0.0 Beta 1 (32-bit) Descargar - FileHorse
Files (UEFI+MBR boot supported) Antivirus 0 / 15 Version 0.097 Size 27.3 MB File Signature Version 1.B7 --> Old Easy2Boot versions download [v1.B7] Easy2Boot Windows 8/10 .exe Installer (Recommended) Antivirus 1 / 15 Version 1.B7 Size 24.7 MB File Signature Old Easy2Boot versions download [v1.B7] Easy2Boot Windows 8/10 .exe Installer (includes 32-bit Windows XP drivers) Antivirus 1 / 15 Version 1.B7 Size 36 MB File Signature Old Easy2Boot versions download [v1.B7] Easy2Boot MPI_Tool_Pack - for making .imgPTN files (UEFI+MBR boot supported) Antivirus 0 / 15 Version 097a Size 27.3 MB File Signature Old Easy2Boot versions download [v1.B7] Easy2Boot ZIP file for Linux and XP users (no XP 32-bit drivers) Antivirus 2 / 15 Version 1.B7 Size 28.7 MB File Signature Old Easy2Boot versions download [v1.B7] Easy2Boot Zip file for Linux and XP users (Includes 32-bit Windows XP drivers) Antivirus 2 / 14 Version 1.B7 Size 57.6 MB File Signature Version 1.B6 --> Old Easy2Boot versions download [v1.B6] Easy2Boot Windows 8/10 Installer (Recommended) Antivirus 1 / 15 Version 2 Size 24.7 MB File Signature Old Easy2Boot versions download [v1.B6] Easy2Boot Windows 8/10 Installer (Includes 32-bit Windows XP drivers) Antivirus 1 / 15 Version 2 Size 36 MB File Signature Old Easy2Boot versions download [v1.B6] Easy2Boot MPI_Tool_Pack - For making .imgPTN files (UEFI+MBR boot supported) Antivirus 0 / 15 Version 097 Size 27.3 MB File Signature Old Easy2Boot versions download [v1.B6] Easy2Boot ZIP file for Linux and XP users (no XP 32-bit drivers) Antivirus 1 / 15 Version 2 Size 28.7 MB File Signature Old Easy2Boot versions download [v1.B6] Easy2Boot Zip file for Linux and XP users (Includes 32-bit Windows XP drivers) Antivirus 1 / 14 Version 2 Size 57.6 MB File Signature Version 1.B5 --> Old Easy2Boot versions download [v1.B5] Easy2Boot Windows Installer (Includes 32-bit Windows XP drivers) Antivirus 1 / 15 Version 1.B5 Size 34.9 MB File Signature Old Easy2Boot versions download [v1.B5] Easy2Boot Windows Installer (Recommended) Antivirus 1 / 15 Version 1.B5 Size 23.6 MB File Signature Old Easy2Boot versions download [v1.B5] Easy2Boot For Linux users (Includes 32-bit Windows XP drivers) Antivirus 1 / 14 Version 1.B5 Size 57.5. Hydrogen 1.0.0 Beta 1 (32-bit) Date released: (6 years ago) Download. Hydrogen 0.9.7 (32-bit) Date released: (7 years ago) Download. Hydrogen 0.9.7 Beta 3 (32-bit) Date released: (8 years ago) Download. Hydrogen 0.9.6 Alpha 1. Hydrogen 1.0.0 Beta 1 (32-bit) Date released: (6 years ago) Download. Hydrogen 0.9.7 (32-bit) Date released: (8 years ago) Download. Hydrogen 0.9.7 Beta 3 (32-bit) Date released: (8 years ago) Download. Hydrogen 0.9.6 Alpha 1.Hydrogen 1.0.0 RC 1 (32-bit) Descargar - FileHorse
× 10-1 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) Calculation:Given binary number is00111110011011010000000000000000Here, sign bit is 0. So, number is positive. 0 01111100 11011010000000000000000 Exponent bits = E = 01111100 = 124 (in decimal)Mantissa bits M = 11011010000000000000000In IEEE-754 format, 32-bit (single precision) (-1)s × 1.M × 2E – 127 = (-1)0 × 1.1101101 × 2124 – 127= 1.1101101 × 2-3= (1 + 2-1 + 2-2 + 2-4 + 2-5 + 2-7) × 2-3= 0.231 = 2.31 × 10-1 ≈ 2.27 × 10-1 In IEEE floating point representation, the hexadecimal number 0xC0000000 corresponds to –3.0–1.0–4.0–2.0Answer (Detailed Solution Below) Option 4 : –2.0 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) Calculation:Binary number is0xC0000000 = (11000000000000000000000000000000)2Here, the sign bit is 1. So, the number is negative. 1 10000000 00000000000000000000000 Exponent bits = E = 10000000 = 128 (in decimal)Mantissa bits M = 00000000000000000000000In IEEE-754 format, 32-bit (single precision)(-1)s × 1.M × 2E – 127= (-1)1 × 1. 0 × 2128 – 127= -1 × 1.0 × 2= -2In IEEE floating-point representation, the hexadecimal number 0xC0000000 corresponds to -2. Let R1 and R2 be two 4-bit registers that store numbers in 2’s complement form. For the operation R1 + R2, which one of the following values of R1 and R2 gives an arithmetic overflow? R1 = 1011 and R2 = 1110R1 = 1100 and R2 = 1010R1 = 0011 and R2 = 0100R1 = 1001 and R2 = 1111Answer (Detailed Solution Below) Option 2 : R1 = 1100 and R2 = 1010 The correct answer is option 2.Concept:Stored numbers in registers R1 and R2 are in 2's complement form. Register size is 4 bits. The range of numbers in 2's complement form is -8 to +7. If R1 + R2, the result is out of the above range, then it is overflow.The given data,Given two four-bit registers R1 and R2.Option 1: R1 = 1011 and R2 = 1110False, R1 = 1 0 1 1 = -(0101)= -5+ R2 = 1 1 1 0 = -(0010)= -2----------------------------------------------- 1 0 0 1 = = -7 Here No overflow occurred, because sign bit is same for (R1 + R2 ).Option 2: R1 = 1100 and R2 = 1010True,R1 = 1 1 0 0 = -(0100)= -4+ R2 = 1 0 1 0 = -(0110)= -6 -------------------------------------------- 0 1 1 0 = = -10 Here Overflow occurred because the sign bit is different for (R1 + R2 ).Option 3: R1 = 0011 and R2 = 0100False,R1 = 0 0 1 1 = +(0011)= +3+ R2 = 0 1 01 0 1 1 0 1 1 0 0 1 1 0 1 1 1 1 - University of Toronto
0x408000000xC08000000x834000000xC85800000Answer (Detailed Solution Below) Option 2 : 0xC0800000 Concept: In IEEE- 754 single precision format, a floating-point number is represented in 32 bits. Sign bit (MSB) Biased Exponent (E’) (8 bits) Normalized Mantissa (M’) (23 bits) Sign bit value 0 means positive number, and 1 means a negative number.The floating-point number can be obtained by formula: ± 1. M × 2(E-127)Data:Content of R1: 0x 42200000 (0x means Hexadecimal notation)Content of R2: 0x C1200000Calculation:Content of R1 in Hex (0x) is 42200000. After converting into binary, it can be represented in IEEE- 754 format as: 0 100 0010 0 010 0000 0000 0000 0000 0000 Sign bit is 0 i.e. the number is positiveBiased Exponent (E’) = 100 0010 0 = 132Normalized Mantissa (M) = 010 0000 0000 0000 0000 0000 = .25Therefore, the number in register R1 = + 1.25 * 2(132-127) = 1.25 × 32 = 40Content of R2 in Hex (0x) is C1200000. After converting into binary, it can be represented in IEEE- 754 format as: 1 100 0001 0 010 0000 0000 0000 0000 0000 Sign bit is 1 i.e. the number is negativeBiased Exponent (E’) = 100 0001 0 = 130Normalized Mantissa (M) = 010 0000 0000 0000 0000 0000 = .25Therefore, the number in register R1 = - 1.25 * 2(130-127) = -1.25 * 8 = -10R3 = R1/R2 = 40/-10 = -4Since the number is negative, Sign bit (MSB) = 1Converting 4 into binary of a floating point gives: (100.0)2Representing it into normalized form gives: (1.000000….) × 22Therefore, Mantissa is 23 bits of all 0sBiased Exponent (E’) = E+ 127 = 2+127 = 129 = (10000001)2It can be represented in IEEE- 754 format as: 1 100 0000 1 000 0000 0000 0000 0000 0000 Converting it into Hex format gives: 0x C0800000 The decimal floating-point number -40.1 represented using IEEE-754 32-bit representation and written in hexadecimal form is _____ 0xC22060000xC20066660xC20060000xC2206666Answer (Detailed Solution Below) Option 4 : 0xC2206666 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) In IEEE-754 format, 32-bit (single precision)(-1)s × 1.M × 2E – 127Calculation:Convert: 40.1 to binaryStep 1: convert 40 2 40 2 20 0 2 10 0 2 5 0 2 2 1 2 1 0 0 1 ↑ (40)10 = (101000)2Step 2: convert .1 to binary0.1 × 2 = 0.2 (0)0.2 × 2 = 0.4 (0)0.4 × 0.2 = 0.8 (0)0.8 × 0.2 = 1.6 (1)0.6 × 0.2 = 1.2 (1)0.2 × 0.2 = 0.4 (0) and so onGiven binary number is(40.1)10 = (101000.000110011001100…)2(40.1)10 = 1.0100 0000 1100 1100 … × 25Signed (1 bit) = 1 (given number is negative)Exponent (8 bit) = 5 + 127 =Lightscanner 32 Software 1 0 0 23
...Download Agisoft Metashape (32-bit) for Windows PC from ... 100% Safe and Secure ✓ Free Download (32-bit/64-bit) Latest ... Windows 7 / Windows 8 / Windows 10 ... 1.6.0 (32-bit) · Agisoft Metashape 1.5.5 (32-bit) · Agisoft PhotoScan ... Agisoft Metashape (PhotoScan) is a high-end professional tool for ...The Sims 3 Full Version Free Download Apk - 4f33ed1b8f ... Agisoft Photoscan Pro 0 8 3b 32 64bit Torrent 36 -> ...Fri Aug 07, 2020 @ 10:06PM by Debbie Tiguelo, Agisoft Photoscan Pro 0 8 3b 32 64bit Torrent Download.. Mon Aug 03, 2020 @ 05:26AM by Debbie Tiguelo ...Posts about Agisoft PhotoScan Professional 32 bit download .. Agisoft photoscan pro 0 8 3b 32bit 64bit .Download agisoft photoscan pro torrent ...Agisoft PhotoScan Pro 0.8.3b (32 64bit).torrent ... Agisoft ... or crack serial keygen cd key etos 4.1 crack Download: Agisoft Photo Scan Pro 0 8.Download Agisoft Metashape (PhotoScan) Pro 1.5.3 Win64 /Linux/macOS full crack for free at ... Agisoft PhotoScan Professional 1 1 0 (x86-x64) + Crack.. .. Ahmet Kanneci Gitar Metodu Pdf 35 e1a097fadcHydrogen (32-bit) - tech-lib.net
Ports for Outputs/Inputs, Optional I/O Board: 6 digital inputs, 5 relays as contact closures, 1 analog Analog Board: 4 or 8 configurable as 4-20mA or 0-20mA Relay Board: 8 or 16 programmable contact closures Operating Temperature 32 to 104°F (0 to 40°C) Operating Humidity 0 to 95% (non-condensing) Configuration Benchtop or 19” (48.3cm) rack-mount, 3U Other Specifications (all models):Physical Specifications 5.12” H x 19” W x 22.4” D (13 cm x 48.26 cm x 56.9 cm) Electrical Requirements Voltage: 100 - 230 VAC 50/60 Hz, 2 A Preventive Maintenance Kit Includes all parts potentially needed on-handBaseline 8990 Permeation Calibrator For use with permeation tubes instead of cylindersBaseline 9130 Sample Conditioner Delivers a particulate and moisture free sampleBaseline 9150 Multipoint Sampler To expand the 9100 GC sample points >8Gas Generators Hydrogen generators Zero Air generators Service & Support Options Start-up and training, 1 day onsite Environmental Monitoring AMC in Cleanrooms Air Quality Monitoring Fugitive Emission Monitoring Toxic Gas Monitoring Indoor Air Quality Leak Detection OEM PID Sensors Industrial Process Analysis & Control Beverage Gas Monitoring Industrial Gas Mixing Control Process Gas Analysis Specialty and Industrial Gas Monitoring Surface Logging Analysis. Hydrogen 1.0.0 Beta 1 (32-bit) Date released: (6 years ago) Download. Hydrogen 0.9.7 (32-bit) Date released: (7 years ago) Download. Hydrogen 0.9.7 Beta 3 (32-bit) Date released: (8 years ago) Download. Hydrogen 0.9.6 Alpha 1.
Crack ImageRecall 5 32 Bit 5 0 1 - ecindisretisla.wixsite.com
De 32 .. Arquivo Jaws 15.0.9023 PTB 64 Bits Pacote de instalação narrativa.exe Na conta do ... Download JAWS for Windows 15.0.9023 32/64-bit with crack Torrent .... Existe la versión de Jaws 15.0.10026, de Junio de 2014, pero, por mis propias pruebas, no funciona con el crack de la 15.0.9023, por lo ... Jaws se ha optimizado para los equipos con sistema operativo Windows 8, y, ... O bien, pueden probar a instalar el Jaws 15 de 64 bits, y aplicar el crack automático de .... JAWS for Windows 15 0 9023 32 64 bit with crack. ... JAWS For Windows 15 0 9023 32 64 Bit With Crack. 0 Reads 0 Votes 1 Part Story. eczaiclamir .... 38 Atalhos que Podem ser Usados no windows.txt. 18/2/18 23:276 KB .... Jaws 15.0.9023 PTB, 32 Bits, Pacote de instalação narrativa.exe. 19/8/14 ... JAWS2019V1904.60.4000x64.zip. 13/5/19 .... talks 2[1][1].51 novo and crack.zip. 29/10/13 .... Crack,de,jaws,para,32,bit,-,con,funcin,tndem!.,Crack,automtico,del,Jaws ... ,Search,Engine.,JAWS,for,Windows,15.0.9023,32/64,bit,with,crack.. Orgfullsite.org//288307-jaws-for-windows-professional-15.0-9023.html JAWS ... Jaws 14.0.9002-rus-32 и 64 bit Jaws двенадцатой версии под 32 разрядную. JAWS for Windows 15. 0. 9023 32 64-bit with crack. Download jaws 18. 0 for free. Office Tools downloads - Freedom Scientific JAWS by Freedom Scientific, Inc.. Download jaws 15.0.9023 32bit x 64bit full crack. BKL-INFOKERJA. Add Comment. Jaws. Jaws 15.0.9023 merupakan jaws yang baru saja dirilis oleh .... JAWS 32-bit Professional can be .... Description: a4c8ef0b3e thepiratebay.se JAWS for Windows 15.0.9023 32/64-bit with crack Applications Windows 3 days .... We're dedicated to reader privacy. We never accept ads. .... Jaws VDownload ESET NOD32 AntiVirus 32-bit .0.-1 for Windows
Staircase: not only are the stair steps set at specific heights but the height between steps is fixed). Finally, Bohr suggested that the energy of light emitted from electrified hydrogen gas was equal to the energy difference of the electron’s energy states:Elight = hν = ΔEelectronThis means that only certain frequencies (and thus, certain wavelengths) of light are emitted. Figure 8.5 “Bohr’s Model of the Hydrogen Atom” shows a model of the hydrogen atom based on Bohr’s ideas.Figure 8.5 Bohr’s Model of the Hydrogen AtomBohr’s description of the hydrogen atom had specific orbits for the electron, which had quantized energies.Bohr’s ideas were useful but were applied only to the hydrogen atom. However, later researchers generalized Bohr’s ideas into a new theory called quantum mechanics, which explains the behaviour of electrons as if they were acting as a wave, not as particles. Quantum mechanics predicts two major things: quantized energies for electrons of all atoms (not just hydrogen) and an organization of electrons within atoms. Electrons are no longer thought of as being randomly distributed around a nucleus or restricted to certain orbits (in that regard, Bohr was wrong). Instead, electrons are collected into groups and subgroups that explain much about the chemical behaviour of the atom.In the quantum-mechanical model of an atom, the state of an electron is described by four quantum numbers, not just the one predicted by Bohr. The first quantum number is called the principal quantum number. Represented by n. (n). The principal quantum number largely determines the energy of an electron. Electrons in the same atom that have the same principal quantum number are said to occupy an electron shell of the atom. The principal quantum number can be any nonzero positive integer: 1, 2, 3, 4,….Within a shell, there may be multiple possible values of the next quantum number, the angular momentum quantum number. Represented by ℓ. (ℓ). The ℓ quantum number has a minor effect on the energy of the electron but also affects the spatial distribution of the electron in three-dimensional space—that is, the shape of an electron’s distribution in space. The value of the ℓ quantum number can be any integer between 0 and n − 1:ℓ = 0, 1, 2,…, n − 1Thus, for a given value of n, there are different possible values of ℓ:If n equalsℓ can be1020 or 130, 1, or 240, 1, 2, or 3and so forth. Electrons within a shell that have the same value of ℓ are said to occupy a subshell in the atom. Commonly, instead of referring to the numerical value of ℓ, a letter represents the value of ℓ (to help distinguish it from the principal quantum number):If ℓ equalsThe letter is0s1p2d3fThe. Hydrogen 1.0.0 Beta 1 (32-bit) Date released: (6 years ago) Download. Hydrogen 0.9.7 (32-bit) Date released: (7 years ago) Download. Hydrogen 0.9.7 Beta 3 (32-bit) Date released: (8 years ago) Download. Hydrogen 0.9.6 Alpha 1.WATER AND HYDROGEN – FORM 1
Word EquationWater = Dihydrogen + DioxygenH2O = H2 + O2 is a Decomposition reaction where two moles of Water [H2O] decomposes into two moles of Dihydrogen [H2] and one mole of Dioxygen [O2]Reaction TypeDecompositionRedoxElectrolysis reactionRedox (Oxidation-Reduction) ReactionH2O = H2 + O2 might be a redox reaction. Reactants Water - H2OHydroxic Acid H₂O [Oh2] Aqua Pure Water Hydrogen Oxide Oxidane Dihydrogen Oxide Products Dihydrogen - H2Molecular Hydrogen Hydrogen Molecule Hydrogen Hydrogen Gas Molecular Hydrogen Gas H₂ Dioxygen - O2Lox Liquid Oxygen Oxygen Gas Triplet Oxygen Diatomic Oxygen Molecular Oxygen Oxygen O₂ ThermodynamicsThermodynamics of the reaction can be calculated using a lookup table.Is the Reaction Exothermic or Endothermic?H2O (g)2 mol-241.818464 kJ/mol483.636928 kJH2 (g)2 mol0 kJ/mol0 kJO2 (g)1 mol0 kJ/mol0 kJΣΔH°f(reactants)-483.636928 kJΣΔH°f(products)0 kJΔH°rxn483.636928 kJΣΔH°f(products) > ΣΔH°f(reactants), so H2O = H2 + O2 is endothermic (absorbs heat).Is the Reaction Exoentropic or Endoentropic?ΔS = Sproducts - Sreactants. If ΔS 0, it is endoentropic.H2O (g)2 mol188.715136 J/(mol K)-377.430272 J/KH2 (g)2 mol130.586824 J/(mol K)261.173648 J/KO2 (g)1 mol205.028552 J/(mol K)205.028552 J/KΣΔS°(reactants)377.430272 J/KΣΔS°(products)466.2022 J/KΔS°rxn88.771928 J/KΣΔS°(products) > ΣΔS°(reactants), so H2O = H2 + O2 is endoentropic (increase in entropy).Is the Reaction Exergonic or Endergonic?ΔG = Gproducts - Greactants. If ΔG 0, it is endergonic.H2O (g)2 mol-228.588656 kJ/mol457.177312 kJH2 (g)2 mol0 kJ/mol0 kJO2 (g)1 mol0 kJ/mol0 kJΣΔG°(reactants)-457.177312 kJΣΔG°(products)0 kJΔG°rxn457.177312 kJΣΔG°(products) > ΣΔG°(reactants), so H2O = H2 + O2 is endergonic (absorbs energy). Instructions To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Compound states [like (s) (aq) or (g)] are not required. You can use parenthesis () or brackets []. How To Balance EquationsBalance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured.Read our article on how to balance chemical equations or ask for help in our chat. Examples Water = Dihydrogen + Dioxygen H2 + O2 + (3.76N2) = H2O + N2 H2 + O2 + (3.76N2) = H2O + O2 + N2 H2 + O2 + Al = Al(OH)3 H2 + O2 + Al = AlH3O3 H2 + O2 + C + Na = NaHCO3 H2 + O2 + C + P = CH2OP H2 + O2 + C = (CH2OH)2 H2 + O2 + C = C12H22O11 HNO2 + CaO = CaN2O4 + H2O Mn(OH)7 + H2SO2 = Mn2(SO2)7Comments
Word EquationDihydrogen + Carbon + Dioxygen = Acetic AcidH2 + C + O2 = HCH3CO2 is a Synthesis reaction where two moles of Dihydrogen [H2], two moles of Carbon [C] and one mole of Dioxygen [O2] combine to form one mole of Acetic Acid [HCH3CO2] Reactants Dihydrogen - H2Molecular Hydrogen Hydrogen Molecule Hydrogen Hydrogen Gas Molecular Hydrogen Gas H₂ Carbon - CElement 6 Dioxygen - O2Lox Liquid Oxygen Oxygen Gas Triplet Oxygen Diatomic Oxygen Molecular Oxygen Oxygen O₂ Products Acetic Acid - HCH3CO2Ch3Cooh E260 Ethanoat Ethanoic Acid Aceticum Acidum Methanecarboxylic Acid Ethoic Acid Ethylic Acid ThermodynamicsThermodynamics of the reaction can be calculated using a lookup table.Is the Reaction Exothermic or Endothermic?H2 (g)2 mol0 kJ/mol-0 kJC (g)2 mol716.681544 kJ/mol-1433.363088 kJO2 (g)1 mol0 kJ/mol-0 kJCH3COOH (l acetic acid)1 mol-484.13064 kJ/mol-484.13064 kJΣΔH°f(reactants)1433.363088 kJΣΔH°f(products)-484.13064 kJΔH°rxn-1917.493728 kJΣΔH°f(reactants) > ΣΔH°f(products), so H2 + C + O2 = HCH3CO2 is exothermic (releases heat).Is the Reaction Exoentropic or Endoentropic?ΔS = Sproducts - Sreactants. If ΔS 0, it is endoentropic.H2 (g)2 mol130.586824 J/(mol K)-261.173648 J/KC (g)2 mol157.9865848 J/(mol K)-315.9731696 J/KO2 (g)1 mol205.028552 J/(mol K)-205.028552 J/KCH3COOH (l acetic acid)1 mol159.8288 J/(mol K)159.8288 J/KΣΔS°(reactants)782.1753696 J/KΣΔS°(products)159.8288 J/KΔS°rxn-622.3465696 J/KΣΔS°(reactants) > ΣΔS°(products), so H2 + C + O2 = HCH3CO2 is exoentropic (decrease in entropy).Is the Reaction Exergonic or Endergonic?ΔG = Gproducts - Greactants. If ΔG 0, it is endergonic.H2 (g)2 mol0 kJ/mol-0 kJC (g)2 mol671.289328 kJ/mol-1342.578656 kJO2 (g)1 mol0 kJ/mol-0 kJCH3COOH (l acetic acid)1 mol-389.9488 kJ/mol-389.9488 kJΣΔG°(reactants)1342.578656 kJΣΔG°(products)-389.9488 kJΔG°rxn-1732.527456 kJΣΔG°(reactants) > ΣΔG°(products), so H2 + C + O2 = HCH3CO2 is exergonic (releases energy). Instructions To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Compound states [like (s) (aq) or (g)]
2025-04-17MQ8-Micropython-ESP32MQ-8 Hydrogen gas sensor module for micropython. This module has been tested on ESP-32Hardware InformationThis module can be used without adding 1.47 kohm resistor to GPIO PinThe default pin used was GPIO36 which labeled as VP on ESP32 boardModify the code in MQ8.get_resistance() method to change the default PinCircuit WiringThe circuit wiring setup was tested on Espressif ESP-32 board on Pin GPIO36 (VP) using 10-bit width ADC mode. During the test Wifi connection of the boardwas still accessible. To extend the ADC GPIO connection without inferring the wifi connection please refer to ESP-32 GPIO manualHere's the connection setupSensorESP-32LabelVCC5VVinGNDGNDGNDA0GPIO36VPAdditional InformationThis software module is a part of Hydrogen Energy research in Wardana Research Group, Dept. of Mechanical Engineering, Universitas Brawijaya, Malang,IndonesiaThis module is freely available for any kind of project in research scale, Minimum Viable Product design, Prototyping, and H2 gas detectionFor Industrial Scale Hydrogen Production the use of this module and MQ-X sensor series is not recommendedResponsible PersonHead of Wardana Research Group : Prof. ING. Wardana,Ph.DSoftware Author : Dr. WIlly Satrio NResearch Manager : Dr. PurnamiContact InfoFor Research Collaboration or Project please email to wardana@ub.ac.id and alternative contact to willy13101307@gmail.comAddressesUniversitas Brawijaya : Address : Jl. Veteran, Ketawanggede, Kec. Lowokwaru, Kota Malang, Jawa Timur 65145Departement : Mechanical EngineeringTerms and ConditionsIf this module is usefull for your research project please cite one of scientific article listed below:Hydrogen production from instant noodle wastewater by organic electrocatalyst coated on PVC surface( external magnetic fields with activated carbon graphene for increasing hydrogen production in water electrolysis( role of turmeric and bicnat on hydrogen production in porous tofu waste suspension electrolysis( effect of curcumin and activated carbon catalyst enhancing hydrogen production from biomass pyrolysis( role of activated carbon in boosting the activity of clitoria ternatea powder photocatalyst for hydrogen production( effect of curcumin coated electrode on hydrogen production through water electrolysis( production by photocatalysis method of glutamic acid and activated carbon( Of Bamboo Charcoal And Fragaria Vesca Powder Photocatalysts In Hydrogen Production Via Water Splitting(DOI : 10.15587/1729-4061.2020.213277)
2025-03-27× 10-1 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) Calculation:Given binary number is00111110011011010000000000000000Here, sign bit is 0. So, number is positive. 0 01111100 11011010000000000000000 Exponent bits = E = 01111100 = 124 (in decimal)Mantissa bits M = 11011010000000000000000In IEEE-754 format, 32-bit (single precision) (-1)s × 1.M × 2E – 127 = (-1)0 × 1.1101101 × 2124 – 127= 1.1101101 × 2-3= (1 + 2-1 + 2-2 + 2-4 + 2-5 + 2-7) × 2-3= 0.231 = 2.31 × 10-1 ≈ 2.27 × 10-1 In IEEE floating point representation, the hexadecimal number 0xC0000000 corresponds to –3.0–1.0–4.0–2.0Answer (Detailed Solution Below) Option 4 : –2.0 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) Calculation:Binary number is0xC0000000 = (11000000000000000000000000000000)2Here, the sign bit is 1. So, the number is negative. 1 10000000 00000000000000000000000 Exponent bits = E = 10000000 = 128 (in decimal)Mantissa bits M = 00000000000000000000000In IEEE-754 format, 32-bit (single precision)(-1)s × 1.M × 2E – 127= (-1)1 × 1. 0 × 2128 – 127= -1 × 1.0 × 2= -2In IEEE floating-point representation, the hexadecimal number 0xC0000000 corresponds to -2. Let R1 and R2 be two 4-bit registers that store numbers in 2’s complement form. For the operation R1 + R2, which one of the following values of R1 and R2 gives an arithmetic overflow? R1 = 1011 and R2 = 1110R1 = 1100 and R2 = 1010R1 = 0011 and R2 = 0100R1 = 1001 and R2 = 1111Answer (Detailed Solution Below) Option 2 : R1 = 1100 and R2 = 1010 The correct answer is option 2.Concept:Stored numbers in registers R1 and R2 are in 2's complement form. Register size is 4 bits. The range of numbers in 2's complement form is -8 to +7. If R1 + R2, the result is out of the above range, then it is overflow.The given data,Given two four-bit registers R1 and R2.Option 1: R1 = 1011 and R2 = 1110False, R1 = 1 0 1 1 = -(0101)= -5+ R2 = 1 1 1 0 = -(0010)= -2----------------------------------------------- 1 0 0 1 = = -7 Here No overflow occurred, because sign bit is same for (R1 + R2 ).Option 2: R1 = 1100 and R2 = 1010True,R1 = 1 1 0 0 = -(0100)= -4+ R2 = 1 0 1 0 = -(0110)= -6 -------------------------------------------- 0 1 1 0 = = -10 Here Overflow occurred because the sign bit is different for (R1 + R2 ).Option 3: R1 = 0011 and R2 = 0100False,R1 = 0 0 1 1 = +(0011)= +3+ R2 = 0 1 0
2025-04-19