Hydrogen 1 0 2 32 bit
Author: c | 2025-04-24

Hydrogen 1.1.1 (32-bit) Date released: (one year ago) Download. Hydrogen 1.1.0 (32-bit) Date released: (2 years ago) Download. Hydrogen 1.0.0 Beta 1 (32-bit)
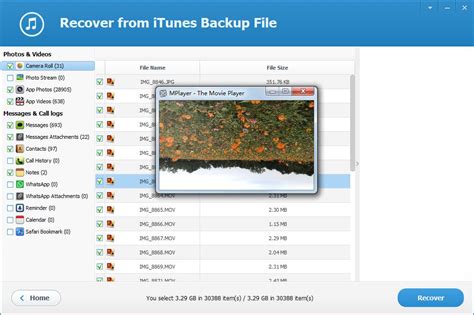
Hydrogen 1.0.0 RC 1 (32-bit) Download - FileHorse
Word EquationDihydrogen + Carbon + Dioxygen = Acetic AcidH2 + C + O2 = HCH3CO2 is a Synthesis reaction where two moles of Dihydrogen [H2], two moles of Carbon [C] and one mole of Dioxygen [O2] combine to form one mole of Acetic Acid [HCH3CO2] Reactants Dihydrogen - H2Molecular Hydrogen Hydrogen Molecule Hydrogen Hydrogen Gas Molecular Hydrogen Gas H₂ Carbon - CElement 6 Dioxygen - O2Lox Liquid Oxygen Oxygen Gas Triplet Oxygen Diatomic Oxygen Molecular Oxygen Oxygen O₂ Products Acetic Acid - HCH3CO2Ch3Cooh E260 Ethanoat Ethanoic Acid Aceticum Acidum Methanecarboxylic Acid Ethoic Acid Ethylic Acid ThermodynamicsThermodynamics of the reaction can be calculated using a lookup table.Is the Reaction Exothermic or Endothermic?H2 (g)2 mol0 kJ/mol-0 kJC (g)2 mol716.681544 kJ/mol-1433.363088 kJO2 (g)1 mol0 kJ/mol-0 kJCH3COOH (l acetic acid)1 mol-484.13064 kJ/mol-484.13064 kJΣΔH°f(reactants)1433.363088 kJΣΔH°f(products)-484.13064 kJΔH°rxn-1917.493728 kJΣΔH°f(reactants) > ΣΔH°f(products), so H2 + C + O2 = HCH3CO2 is exothermic (releases heat).Is the Reaction Exoentropic or Endoentropic?ΔS = Sproducts - Sreactants. If ΔS 0, it is endoentropic.H2 (g)2 mol130.586824 J/(mol K)-261.173648 J/KC (g)2 mol157.9865848 J/(mol K)-315.9731696 J/KO2 (g)1 mol205.028552 J/(mol K)-205.028552 J/KCH3COOH (l acetic acid)1 mol159.8288 J/(mol K)159.8288 J/KΣΔS°(reactants)782.1753696 J/KΣΔS°(products)159.8288 J/KΔS°rxn-622.3465696 J/KΣΔS°(reactants) > ΣΔS°(products), so H2 + C + O2 = HCH3CO2 is exoentropic (decrease in entropy).Is the Reaction Exergonic or Endergonic?ΔG = Gproducts - Greactants. If ΔG 0, it is endergonic.H2 (g)2 mol0 kJ/mol-0 kJC (g)2 mol671.289328 kJ/mol-1342.578656 kJO2 (g)1 mol0 kJ/mol-0 kJCH3COOH (l acetic acid)1 mol-389.9488 kJ/mol-389.9488 kJΣΔG°(reactants)1342.578656 kJΣΔG°(products)-389.9488 kJΔG°rxn-1732.527456 kJΣΔG°(reactants) > ΣΔG°(products), so H2 + C + O2 = HCH3CO2 is exergonic (releases energy). Instructions To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Compound states [like (s) (aq) or (g)]. Hydrogen 1.1.1 (32-bit) Date released: (one year ago) Download. Hydrogen 1.1.0 (32-bit) Date released: (2 years ago) Download. Hydrogen 1.0.0 Beta 1 (32-bit) Hydrogen 1.0.0 (32-bit) Date released: (3 years ago) Hydrogen 1.0.0 RC 1 (32-bit) Date released: (3 years ago) Download. Hydrogen 1.0.0 Beta 2 (32-bit) Date Hydrogen 1.0.0 (32-bit) Date released: (4 years ago) Hydrogen 1.0.0 RC 1 (32-bit) Date released: (4 years ago) Download. Hydrogen 1.0.0 Beta 2 (32-bit) Date Hydrogen 1.0.0 Beta 2 (32-bit) Date released: (4 years ago) Download. Hydrogen 1.0.0 Beta 1 (32-bit) Date released: (6 years ago) Download. Hydrogen 0.9.7 (32-bit) Date released: (8 years ago) Download. Hydrogen 0.9.7 Beta 3 (32-bit) Hydrogen 1.0.0 Beta 2 (32-bit) Date released: (4 years ago) Download. Hydrogen 1.0.0 Beta 1 (32-bit) Date released: (6 years ago) Download. Hydrogen 0.9.7 (32-bit) Date released: (7 years ago) Download. Hydrogen 0.9.7 Beta 3 (32-bit) Hydrogen 1.0.0 RC 1 (32-bit) Date released: (3 years ago) Download. Hydrogen 1.0.0 Beta 2 (32-bit) Date released: (4 years ago) Download. Hydrogen 1.0.0 Hydrogen 1.0.0 RC 1 (32-bit) Date released: (4 years ago) Download. Hydrogen 1.0.0 Beta 2 (32-bit) Date released: (4 years ago) Download. Hydrogen 1.0.0 0x408000000xC08000000x834000000xC85800000Answer (Detailed Solution Below) Option 2 : 0xC0800000 Concept: In IEEE- 754 single precision format, a floating-point number is represented in 32 bits. Sign bit (MSB) Biased Exponent (E’) (8 bits) Normalized Mantissa (M’) (23 bits) Sign bit value 0 means positive number, and 1 means a negative number.The floating-point number can be obtained by formula: ± 1. M × 2(E-127)Data:Content of R1: 0x 42200000 (0x means Hexadecimal notation)Content of R2: 0x C1200000Calculation:Content of R1 in Hex (0x) is 42200000. After converting into binary, it can be represented in IEEE- 754 format as: 0 100 0010 0 010 0000 0000 0000 0000 0000 Sign bit is 0 i.e. the number is positiveBiased Exponent (E’) = 100 0010 0 = 132Normalized Mantissa (M) = 010 0000 0000 0000 0000 0000 = .25Therefore, the number in register R1 = + 1.25 * 2(132-127) = 1.25 × 32 = 40Content of R2 in Hex (0x) is C1200000. After converting into binary, it can be represented in IEEE- 754 format as: 1 100 0001 0 010 0000 0000 0000 0000 0000 Sign bit is 1 i.e. the number is negativeBiased Exponent (E’) = 100 0001 0 = 130Normalized Mantissa (M) = 010 0000 0000 0000 0000 0000 = .25Therefore, the number in register R1 = - 1.25 * 2(130-127) = -1.25 * 8 = -10R3 = R1/R2 = 40/-10 = -4Since the number is negative, Sign bit (MSB) = 1Converting 4 into binary of a floating point gives: (100.0)2Representing it into normalized form gives: (1.000000….) × 22Therefore, Mantissa is 23 bits of all 0sBiased Exponent (E’) = E+ 127 = 2+127 = 129 = (10000001)2It can be represented in IEEE- 754 format as: 1 100 0000 1 000 0000 0000 0000 0000 0000 Converting it into Hex format gives: 0x C0800000 The decimal floating-point number -40.1 represented using IEEE-754 32-bit representation and written in hexadecimal form is _____ 0xC22060000xC20066660xC20060000xC2206666Answer (Detailed Solution Below) Option 4 : 0xC2206666 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) In IEEE-754 format, 32-bit (single precision)(-1)s × 1.M × 2E – 127Calculation:Convert: 40.1 to binaryStep 1: convert 40 2 40 2 20 0 2 10 0 2 5 0 2 2 1 2 1 0 0 1 ↑ (40)10 = (101000)2Step 2: convert .1 to binary0.1 × 2 = 0.2 (0)0.2 × 2 = 0.4 (0)0.4 × 0.2 = 0.8 (0)0.8 × 0.2 = 1.6 (1)0.6 × 0.2 = 1.2 (1)0.2 × 0.2 = 0.4 (0) and so onGiven binary number is(40.1)10 = (101000.000110011001100…)2(40.1)10 = 1.0100 0000 1100 1100 … × 25Signed (1 bit) = 1 (given number is negative)Exponent (8 bit) = 5 + 127 =Comments
Word EquationDihydrogen + Carbon + Dioxygen = Acetic AcidH2 + C + O2 = HCH3CO2 is a Synthesis reaction where two moles of Dihydrogen [H2], two moles of Carbon [C] and one mole of Dioxygen [O2] combine to form one mole of Acetic Acid [HCH3CO2] Reactants Dihydrogen - H2Molecular Hydrogen Hydrogen Molecule Hydrogen Hydrogen Gas Molecular Hydrogen Gas H₂ Carbon - CElement 6 Dioxygen - O2Lox Liquid Oxygen Oxygen Gas Triplet Oxygen Diatomic Oxygen Molecular Oxygen Oxygen O₂ Products Acetic Acid - HCH3CO2Ch3Cooh E260 Ethanoat Ethanoic Acid Aceticum Acidum Methanecarboxylic Acid Ethoic Acid Ethylic Acid ThermodynamicsThermodynamics of the reaction can be calculated using a lookup table.Is the Reaction Exothermic or Endothermic?H2 (g)2 mol0 kJ/mol-0 kJC (g)2 mol716.681544 kJ/mol-1433.363088 kJO2 (g)1 mol0 kJ/mol-0 kJCH3COOH (l acetic acid)1 mol-484.13064 kJ/mol-484.13064 kJΣΔH°f(reactants)1433.363088 kJΣΔH°f(products)-484.13064 kJΔH°rxn-1917.493728 kJΣΔH°f(reactants) > ΣΔH°f(products), so H2 + C + O2 = HCH3CO2 is exothermic (releases heat).Is the Reaction Exoentropic or Endoentropic?ΔS = Sproducts - Sreactants. If ΔS 0, it is endoentropic.H2 (g)2 mol130.586824 J/(mol K)-261.173648 J/KC (g)2 mol157.9865848 J/(mol K)-315.9731696 J/KO2 (g)1 mol205.028552 J/(mol K)-205.028552 J/KCH3COOH (l acetic acid)1 mol159.8288 J/(mol K)159.8288 J/KΣΔS°(reactants)782.1753696 J/KΣΔS°(products)159.8288 J/KΔS°rxn-622.3465696 J/KΣΔS°(reactants) > ΣΔS°(products), so H2 + C + O2 = HCH3CO2 is exoentropic (decrease in entropy).Is the Reaction Exergonic or Endergonic?ΔG = Gproducts - Greactants. If ΔG 0, it is endergonic.H2 (g)2 mol0 kJ/mol-0 kJC (g)2 mol671.289328 kJ/mol-1342.578656 kJO2 (g)1 mol0 kJ/mol-0 kJCH3COOH (l acetic acid)1 mol-389.9488 kJ/mol-389.9488 kJΣΔG°(reactants)1342.578656 kJΣΔG°(products)-389.9488 kJΔG°rxn-1732.527456 kJΣΔG°(reactants) > ΣΔG°(products), so H2 + C + O2 = HCH3CO2 is exergonic (releases energy). Instructions To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Compound states [like (s) (aq) or (g)]
2025-04-210x408000000xC08000000x834000000xC85800000Answer (Detailed Solution Below) Option 2 : 0xC0800000 Concept: In IEEE- 754 single precision format, a floating-point number is represented in 32 bits. Sign bit (MSB) Biased Exponent (E’) (8 bits) Normalized Mantissa (M’) (23 bits) Sign bit value 0 means positive number, and 1 means a negative number.The floating-point number can be obtained by formula: ± 1. M × 2(E-127)Data:Content of R1: 0x 42200000 (0x means Hexadecimal notation)Content of R2: 0x C1200000Calculation:Content of R1 in Hex (0x) is 42200000. After converting into binary, it can be represented in IEEE- 754 format as: 0 100 0010 0 010 0000 0000 0000 0000 0000 Sign bit is 0 i.e. the number is positiveBiased Exponent (E’) = 100 0010 0 = 132Normalized Mantissa (M) = 010 0000 0000 0000 0000 0000 = .25Therefore, the number in register R1 = + 1.25 * 2(132-127) = 1.25 × 32 = 40Content of R2 in Hex (0x) is C1200000. After converting into binary, it can be represented in IEEE- 754 format as: 1 100 0001 0 010 0000 0000 0000 0000 0000 Sign bit is 1 i.e. the number is negativeBiased Exponent (E’) = 100 0001 0 = 130Normalized Mantissa (M) = 010 0000 0000 0000 0000 0000 = .25Therefore, the number in register R1 = - 1.25 * 2(130-127) = -1.25 * 8 = -10R3 = R1/R2 = 40/-10 = -4Since the number is negative, Sign bit (MSB) = 1Converting 4 into binary of a floating point gives: (100.0)2Representing it into normalized form gives: (1.000000….) × 22Therefore, Mantissa is 23 bits of all 0sBiased Exponent (E’) = E+ 127 = 2+127 = 129 = (10000001)2It can be represented in IEEE- 754 format as: 1 100 0000 1 000 0000 0000 0000 0000 0000 Converting it into Hex format gives: 0x C0800000 The decimal floating-point number -40.1 represented using IEEE-754 32-bit representation and written in hexadecimal form is _____ 0xC22060000xC20066660xC20060000xC2206666Answer (Detailed Solution Below) Option 4 : 0xC2206666 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) In IEEE-754 format, 32-bit (single precision)(-1)s × 1.M × 2E – 127Calculation:Convert: 40.1 to binaryStep 1: convert 40 2 40 2 20 0 2 10 0 2 5 0 2 2 1 2 1 0 0 1 ↑ (40)10 = (101000)2Step 2: convert .1 to binary0.1 × 2 = 0.2 (0)0.2 × 2 = 0.4 (0)0.4 × 0.2 = 0.8 (0)0.8 × 0.2 = 1.6 (1)0.6 × 0.2 = 1.2 (1)0.2 × 0.2 = 0.4 (0) and so onGiven binary number is(40.1)10 = (101000.000110011001100…)2(40.1)10 = 1.0100 0000 1100 1100 … × 25Signed (1 bit) = 1 (given number is negative)Exponent (8 bit) = 5 + 127 =
2025-04-10MQ8-Micropython-ESP32MQ-8 Hydrogen gas sensor module for micropython. This module has been tested on ESP-32Hardware InformationThis module can be used without adding 1.47 kohm resistor to GPIO PinThe default pin used was GPIO36 which labeled as VP on ESP32 boardModify the code in MQ8.get_resistance() method to change the default PinCircuit WiringThe circuit wiring setup was tested on Espressif ESP-32 board on Pin GPIO36 (VP) using 10-bit width ADC mode. During the test Wifi connection of the boardwas still accessible. To extend the ADC GPIO connection without inferring the wifi connection please refer to ESP-32 GPIO manualHere's the connection setupSensorESP-32LabelVCC5VVinGNDGNDGNDA0GPIO36VPAdditional InformationThis software module is a part of Hydrogen Energy research in Wardana Research Group, Dept. of Mechanical Engineering, Universitas Brawijaya, Malang,IndonesiaThis module is freely available for any kind of project in research scale, Minimum Viable Product design, Prototyping, and H2 gas detectionFor Industrial Scale Hydrogen Production the use of this module and MQ-X sensor series is not recommendedResponsible PersonHead of Wardana Research Group : Prof. ING. Wardana,Ph.DSoftware Author : Dr. WIlly Satrio NResearch Manager : Dr. PurnamiContact InfoFor Research Collaboration or Project please email to wardana@ub.ac.id and alternative contact to willy13101307@gmail.comAddressesUniversitas Brawijaya : Address : Jl. Veteran, Ketawanggede, Kec. Lowokwaru, Kota Malang, Jawa Timur 65145Departement : Mechanical EngineeringTerms and ConditionsIf this module is usefull for your research project please cite one of scientific article listed below:Hydrogen production from instant noodle wastewater by organic electrocatalyst coated on PVC surface( external magnetic fields with activated carbon graphene for increasing hydrogen production in water electrolysis( role of turmeric and bicnat on hydrogen production in porous tofu waste suspension electrolysis( effect of curcumin and activated carbon catalyst enhancing hydrogen production from biomass pyrolysis( role of activated carbon in boosting the activity of clitoria ternatea powder photocatalyst for hydrogen production( effect of curcumin coated electrode on hydrogen production through water electrolysis( production by photocatalysis method of glutamic acid and activated carbon( Of Bamboo Charcoal And Fragaria Vesca Powder Photocatalysts In Hydrogen Production Via Water Splitting(DOI : 10.15587/1729-4061.2020.213277)
2025-04-04× 10-1 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) Calculation:Given binary number is00111110011011010000000000000000Here, sign bit is 0. So, number is positive. 0 01111100 11011010000000000000000 Exponent bits = E = 01111100 = 124 (in decimal)Mantissa bits M = 11011010000000000000000In IEEE-754 format, 32-bit (single precision) (-1)s × 1.M × 2E – 127 = (-1)0 × 1.1101101 × 2124 – 127= 1.1101101 × 2-3= (1 + 2-1 + 2-2 + 2-4 + 2-5 + 2-7) × 2-3= 0.231 = 2.31 × 10-1 ≈ 2.27 × 10-1 In IEEE floating point representation, the hexadecimal number 0xC0000000 corresponds to –3.0–1.0–4.0–2.0Answer (Detailed Solution Below) Option 4 : –2.0 Concept:32-bit floating-point representation of a binary number in IEEE- 754 is Sign (1 bit) Exponent (8 bit) Mantissa bit (23 bits) Calculation:Binary number is0xC0000000 = (11000000000000000000000000000000)2Here, the sign bit is 1. So, the number is negative. 1 10000000 00000000000000000000000 Exponent bits = E = 10000000 = 128 (in decimal)Mantissa bits M = 00000000000000000000000In IEEE-754 format, 32-bit (single precision)(-1)s × 1.M × 2E – 127= (-1)1 × 1. 0 × 2128 – 127= -1 × 1.0 × 2= -2In IEEE floating-point representation, the hexadecimal number 0xC0000000 corresponds to -2. Let R1 and R2 be two 4-bit registers that store numbers in 2’s complement form. For the operation R1 + R2, which one of the following values of R1 and R2 gives an arithmetic overflow? R1 = 1011 and R2 = 1110R1 = 1100 and R2 = 1010R1 = 0011 and R2 = 0100R1 = 1001 and R2 = 1111Answer (Detailed Solution Below) Option 2 : R1 = 1100 and R2 = 1010 The correct answer is option 2.Concept:Stored numbers in registers R1 and R2 are in 2's complement form. Register size is 4 bits. The range of numbers in 2's complement form is -8 to +7. If R1 + R2, the result is out of the above range, then it is overflow.The given data,Given two four-bit registers R1 and R2.Option 1: R1 = 1011 and R2 = 1110False, R1 = 1 0 1 1 = -(0101)= -5+ R2 = 1 1 1 0 = -(0010)= -2----------------------------------------------- 1 0 0 1 = = -7 Here No overflow occurred, because sign bit is same for (R1 + R2 ).Option 2: R1 = 1100 and R2 = 1010True,R1 = 1 1 0 0 = -(0100)= -4+ R2 = 1 0 1 0 = -(0110)= -6 -------------------------------------------- 0 1 1 0 = = -10 Here Overflow occurred because the sign bit is different for (R1 + R2 ).Option 3: R1 = 0011 and R2 = 0100False,R1 = 0 0 1 1 = +(0011)= +3+ R2 = 0 1 0
2025-03-28Word EquationWater = Dihydrogen + DioxygenH2O = H2 + O2 is a Decomposition reaction where two moles of Water [H2O] decomposes into two moles of Dihydrogen [H2] and one mole of Dioxygen [O2]Reaction TypeDecompositionRedoxElectrolysis reactionRedox (Oxidation-Reduction) ReactionH2O = H2 + O2 might be a redox reaction. Reactants Water - H2OHydroxic Acid H₂O [Oh2] Aqua Pure Water Hydrogen Oxide Oxidane Dihydrogen Oxide Products Dihydrogen - H2Molecular Hydrogen Hydrogen Molecule Hydrogen Hydrogen Gas Molecular Hydrogen Gas H₂ Dioxygen - O2Lox Liquid Oxygen Oxygen Gas Triplet Oxygen Diatomic Oxygen Molecular Oxygen Oxygen O₂ ThermodynamicsThermodynamics of the reaction can be calculated using a lookup table.Is the Reaction Exothermic or Endothermic?H2O (g)2 mol-241.818464 kJ/mol483.636928 kJH2 (g)2 mol0 kJ/mol0 kJO2 (g)1 mol0 kJ/mol0 kJΣΔH°f(reactants)-483.636928 kJΣΔH°f(products)0 kJΔH°rxn483.636928 kJΣΔH°f(products) > ΣΔH°f(reactants), so H2O = H2 + O2 is endothermic (absorbs heat).Is the Reaction Exoentropic or Endoentropic?ΔS = Sproducts - Sreactants. If ΔS 0, it is endoentropic.H2O (g)2 mol188.715136 J/(mol K)-377.430272 J/KH2 (g)2 mol130.586824 J/(mol K)261.173648 J/KO2 (g)1 mol205.028552 J/(mol K)205.028552 J/KΣΔS°(reactants)377.430272 J/KΣΔS°(products)466.2022 J/KΔS°rxn88.771928 J/KΣΔS°(products) > ΣΔS°(reactants), so H2O = H2 + O2 is endoentropic (increase in entropy).Is the Reaction Exergonic or Endergonic?ΔG = Gproducts - Greactants. If ΔG 0, it is endergonic.H2O (g)2 mol-228.588656 kJ/mol457.177312 kJH2 (g)2 mol0 kJ/mol0 kJO2 (g)1 mol0 kJ/mol0 kJΣΔG°(reactants)-457.177312 kJΣΔG°(products)0 kJΔG°rxn457.177312 kJΣΔG°(products) > ΣΔG°(reactants), so H2O = H2 + O2 is endergonic (absorbs energy). Instructions To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Compound states [like (s) (aq) or (g)] are not required. You can use parenthesis () or brackets []. How To Balance EquationsBalance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured.Read our article on how to balance chemical equations or ask for help in our chat. Examples Water = Dihydrogen + Dioxygen H2 + O2 + (3.76N2) = H2O + N2 H2 + O2 + (3.76N2) = H2O + O2 + N2 H2 + O2 + Al = Al(OH)3 H2 + O2 + Al = AlH3O3 H2 + O2 + C + Na = NaHCO3 H2 + O2 + C + P = CH2OP H2 + O2 + C = (CH2OH)2 H2 + O2 + C = C12H22O11 HNO2 + CaO = CaN2O4 + H2O Mn(OH)7 + H2SO2 = Mn2(SO2)7
2025-04-14